Important Compounds of Group 16 Elements
Important Compounds of Group 16 Elements: Overview
This Topic covers sub-topics such as Ozone, Sulphuric Acid, Sulphur Dioxide, Oleum, Sodium Thiosulphate, Structure of Sulphur Dioxide, Depletion of Ozone Layer, Structure of Ozone and, Uses of Sulphuric Acid
Important Questions on Important Compounds of Group 16 Elements
Favourable conditions for the high production of by catalytic oxidation of with which is the key step in the Contact process of manufacture of are
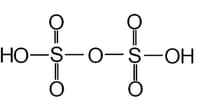
The chemical formula of _____ (soda/oleum) is .
The chemical formula of oleum is .
The structure is shown below is of _____ (oleum/soda).
Write the chemical formula of oleum.
The acid which has peroxy linkage is
Sulphuric acid is
_____ gas is evolved in the thermal decomposition of potassium chlorate.
The products formed in the thermal decomposition of potassium chlorate in the presence of a catalyst are:
On addition of concentrated to a chloride salt, colourless fumes are evolved but in case of an iodide salt, violet fumes come out. This is because
Moist hydrogen cannot be dried over concentrated because it:
Industrially sulphuric acid is manufactured by-
Common name of concentrated sulphuric acid is-
The acid, which does not contain peroxy linkage, is
Identify a neutral oxide from the following oxides.
Bleaching action of is due to :
Moist hydrogen cannot be dried over concentrated because it:
Which of the following reactions is incorrect?
Which of the following is used as disinfectant?
Which of the statements below is/are correct?
(i) Ozone is a toxic gas.
(ii) Ozone is a strong oxidising agent.
(iii) Ozone cannot react with unburnt hydrocarbons in the air.